
Nanoscale Assembly and
Electron Microscopy Lab
Department of Chemical and Biomolecular Engineering
University of Maryland
Liquid Phase Transmission Electron Microscopy
Liquid phase transmission electron microscopy (LP-TEM) is a technique for imaging hydrated nanoscale samples in their native environment with transmission electron microscopy (top). The strength of LP-TEM is its ability to unambiguously reveal nanoscale mechanisms for dynamic processes for nearly any type of nanosized hydrated material, including nanomaterials, live cells, biomolecules, and energy-storage materials. Electrochemical capabilities enable investigation of battery electrode materials and electrocatalysis. The technique is capable of up to video-rate imaging to form in situ movies of nanoscale dynamics (e.g. see movies below).Liquid cell electron microscopy collaborations are welcome, interested parties should contact Prof. Woehl.


Nanochemistry of complex nanocrystal synthesis
Metal nanocrystals are small particles of metal millions of times smaller than the diamter of a human hair. They have critical applications in accelerating chemical reactions (oil refining) and electrochemical reactions (hydrogel fuel cells, electrolyzers). They are typically synthesized with solution chemistry from molecular precursors. Mixing multiple metals into each nanocrystal can significnatly enhance their performance, but the complex reaction mechanisms underlying the conversion of precursor molecules into nanocrystals remain poorly understood. This research area in the Woehl lab uses advanced electron microscopy to uncover these reaction mechanisms by directly watching nanocrystals form in solution with near atomic resolution. This work is currently supported by ACS PRF and an NSF CAREER award.


(Left) Schematic cartoon of LCEM and mass spectrometry for establishing the growth mechanism of high entropy alloy nanoparticles (Right) Atomic resolution images and mass spectrum of high entropy alloy nanoparticles.
Nature and biology inspired assembly of soft matter
Soft matter is a class of materials that are bonded by weak non-covalent bonds that enables them to be responsive and reconfiged by external stimuli (light, pH, electric/magnetic field). Biological cells utilize chemical reactions assembly biomolecules into functional soft matter, such as microtubules or actin filaments, that facilitate cell division and transport of nutrients. Discovery of synthetic systems that exhibit similar behavior is of current interest due to their ability to autonomously change their physical properties (e.g. mechanical and optical properties) and perform functions like sensing and catalysis in response to addition of a chemical fuel. The overall goal of the project is to establish the mechanisms for how chemical reactions drive the assembly of nanoparticles, colloids, and protein hydrogels. This work is currently supported by the Army Research Office.



(Left) Schematic of microtubules that are formed by reactions between proteins and GTP. (Center) Reaction mechanism for microtubule formation and collapse. (Right) Schematic showing how synthetic (not biological) redox reactions can be used to formation transient hydrogels from protein molecules.
Nanoscale transport processes in atmospheric aerosols


(Left) Complex processes occur in aerosols and aerosol droplets. (Right) Schematic showing use of in situ TEM to visualize aerosol phase processes at the nanoscale.
Atmospheric aerosols are critical to understand for public health, climate change, and weather patterns. Atmospheric aerosols are nanoparticles composed of materials such as salts (from sea spray), organic materials (from forest fires, cooking, chemical processing) and biological molecules and entities (viruses, bacteria, and proteins). Conventional methods for investigating the properties of aerosols involve measurements on billions of aerosol particles followed by ensemble averaging of the results. For this reason, it has been challenging to understand the properties of complex aerosol particles that contain multiple types of materials mixed together. Further, conventional methods cannot probe dynamic processes occurring in aerosols, such as water absorption or aggregation. This project focuses on developing new electron microscopy tools for imaging aerosol nanoparticles with nanometer scale spatial resolution. Current work focuses on understanding how aerosol particles act as seeds for water condensation during cloud formation (cloud condensation nuclei). This work is currently supported by the National Science Foundation in collaboration with the Asa-Awuku lab at University of Maryland.
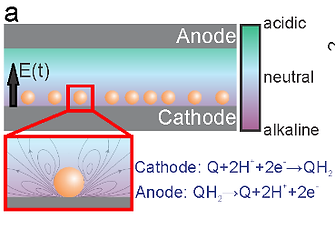
Electrokinetics of colloids in pH gradients
This project studies the behavior of micron scale colloids in pH gradients and low frequency (< 1 kHz) oscillatory electric fields. Microscopic pH gradients are electrochemically generated near the electrode surface by redox reactions of electroactive quinone molecules, which consume or generate protons at the electrode-electrolyte interface. The behavior of the colloids in a parallel plate electrochemical cell is directly observed with optical and confocal microscopy. Colloids experience various competitive and synergistic phoretic and advective forces, including electrophoresis, electroosmosis, electrohydrodynamic fluid flow, and sedimentation, which together determine the assembly state and levitation height of colloids above the electrode surface. Dynamic coupling of colloid surface charge and dipole field as the particles move through the pH gradient lead to interesting behavior, including explosive disassembly of colloidal crystals, particle levitation, and separation of particles based on their size, shape, density, and surface chemistry. We are currently exploring research questions including, What is the effect of dynamic colloid surface charge on AC and DC electrokinetic phenomena (e.g. electrophoresis, electrohydrodynamic flow)? How do multiple electrokinetic phenomena work competitively and synergistically to manipulate colloids in pH gradients? New electrokinetic phenomena discovered in this project will have applications in separating mixtures of similarly sized particles based on shape (e.g. mixtures of colloidal oligomers) and surface chemistry.

Funding




